| United States, California | |
| Orange County Heart Institute | |
| Orange, California, United States, 92868 | |
| United States, Florida | |
| Cardiac Arrhythmia Service | |
| Boca Raton, Florida, United States, 33432 | |
| United States, Georgia | |
| Gwinnett Medical Center | |
| Lawrenceville, Georgia, United States, 30046 | |
| United States, Kentucky | |
| Lexington Cardiology Consultants | |
| Lexington, Kentucky, United States, 40503 | |
| United States, Michigan | |
| Cardiology Institute of Michigan | |
| Flint, Michigan, United States, 48507 | |
| United States, New York | |
| SUNY Downstate | |
| Brooklyn, New York, United States, 11203 | |
| United States, Oklahoma | |
| Oklahoma Heart Hospital | |
| Oklahoma City, Oklahoma, United States, 73120 | |
| United States, Tennessee | |
| Erlanger Clinical Research | |
| Chattanooga, Tennessee, United States, 37403 | |
| Methodist Healthcare Foundation | |
| Memphis, Tennessee, United States, 38104 | |
| United States, Virginia | |
| VA Beach General | |
| Virginia Beach, Virginia, United States, 23454 | |
| United States, Washington | |
| Swedish Medical Center | |
| Seattle, Washington, United States, 98122 | |
| United States, West Virginia | |
| CAMC | |
| Charleston, West Virginia, United States, 25304 | |
| St. Mary's Medical Center | |
| Huntington, West Virginia, United States, 25702 | |
| Austria | |
| Klinische Abteilung für Kardiologie | |
| Graz, Austria, 8036 | |
| Germany | |
| Universitats-Herzzentrum Freiburg - Bad Krozingen | |
| Bad Krozingen, Germany, 79189 | |
| Kerckhoff-Klinik | |
| Bad Nauheim, Germany | |
| Medizinische Klinik & Poliklinik II - Kardiologie | |
| Bonn, Germany, 53105 | |
| Klinikum Coburg | |
| Coburg, Germany, 96450 | |
| Department für Herzinsuffizienz und Devicetherap | |
| Hamburg, Germany | |
| St. Vinzenz Hospital | |
| Köln, Germany, 50733 | |
| Klinik für Innere Medizin III | |
| Villingen-Schwenningen, Germany, 78052 | |
Zoll Life Vest Model 4000 Manual
Abstract
Zoll Lifevest 4000 Manual (b)(4) complaints (b)(4) that reasonably suggest that Life Vest, model #4000, may have caused or contributed to a death or serious injury, were not thoroughly. Medical Policy Wearable Cardioverter Defibrillator (WCD) Effective Date: November, 2007. The LifeVest is designed to perform the same functions as an automatic implantable cardioverter defibrillator (ICD), but is worn outside the body and therefore is noninvasive.
It is well established that implantable cardioverter defibrillator (ICD) is a life saving device ensuring protection against life threatening ventricular arrhythmias. But there are certain situations like a recent myocardial infarction where the standard guidelines do not recommend the implantation of an ICD while the patient can still be at a risk of demise due to a life threatening ventricular arrhythmia. There could also be a temporary indication for protection while explanting an infected ICD system. The wearable cardioverter defibrillator (WCD) is a device which comes to the rescue in such situations. In this brief review, we discuss the historical aspects of the development of a WCD, technical aspects as well as the clinical trial data and real world scenario of its use.
1. Introduction
It is well established that the implantable cardioverter defibrillator (ICD) is a life saving device, especially in patients with a previous myocardial infarction and reduced ejection fraction.1 But the DINAMIT study2 showed that prophylactic ICD implantation is not useful in patients with recent myocardial infarction. Still every clinician would have anecdotal experience of patients who have had sudden cardiac death (SCD) after a recent myocardial infarction and VALIANT (Valsartan in Acute Myocardial Infarction) study showed that the risk of SCD in post myocardial infarction patients with left ventricular dysfunction or heart failure is highest in the first 30 days after the event.3 The wearable cardioverter defibrillator (WCD) (LifeVest, ZOLL, Pittsburgh, Pennsylvania) is a device which can be used to bridge the situation when a patient is waiting for an ICD. This could be either a patient with recent myocardial infarction and left ventricular dysfunction within the period of forty days when the definitive indication for ICD is not yet established or when ICD implantation needs to be deferred in patients with surgical contraindication (i.e. infection, vascular obstruction, treatable comorbidities). In this review we will examine the technical details of a WCD as well the current evidence for its clinical use since it is a relatively new introduction.
I chose “TrialPay” and it was absolutely free as I could cancel two days later with no charge. Samsung mobile unlock software. I give it 10/10. Found some mixed reviews, but I must say: I found it extremely easy to use! I would recommend this site to anyone whether you are wanting a free unlock or willing to pay for your unlock code!
2. Historical aspects
In 1998, Angelo Auricchio and asssociates4 published the preliminary data on the use of WCD in 15 persons who had survived a cardiac arrest due to ventricular tachycardia (VT)/ventricular fibrillation (VF). The WCD had four sensing electrodes and three defibrillation pads integrated into garment to be worn by the patient. The study was conducted in the electrophysiology laboratory under conscious sedation. The defibrillator device had a maximum capacity of 285 Joules (J) monophasic shock. Though the device was capable of automatic sensing and discharging, manual charging and discharging was used in this study to demonstrate the effectiveness of a 230 J shock to terminate an induced VT/VF episode. A single 230 J shock was successful in all the 10 cases in which a VF/fast VT was inducible during the study. The arrhythmia was correctly detected in nine of the ten cases while it was not detected in one case due to the erroneous disconnection of the sensing electrodes at the time of arrhythmia induction.
While the initial report was an acute evaluation of the efficacy of the WCD within the limits of an electrophysiology laboratory, the next one evaluated the efficacy in the field.5 The WCD tested was a vest with ECG monitoring and defibrillator electrodes along with a monitor and an alarm system. The home based interrogation device was connected to the hospital through a modem. The WCD used had a weight of approximately 1500 g and a maximum energy output of 285 J. Of the 39 patients reported, six had ventricular fibrillation in the setting of acute myocardial infarction while 17 had left ventricular ejection fraction (LVEF) of less than 30% and 16 had non-sustained ventricular tachycardia (NSVT). Patients were provided two to three days in hospital training for the use of the device and adaptation. Three of four episodes of VT/VF were correctly identified and terminated. Two of these patients eventually received an ICD. Noteworthy, none of the patients had an inappropriate WCD discharge, though artifactual alarms occurred in 15%. All NSVTs were promptly recognized, but defibrillator discharge was withheld by the patients.
United States Food and Drug Administration (FDA) approval for the first WCD from Lifecor Inc. of Pittsburgh was obtained in 2002.6 As per the FDA Consumer Magazine, March–April 2002, the device was to be worn 24 h a day, except during bathing or showering. User had to transfer the data to the monitoring hospital usually once a week using the modem. FDA had on its file, data from 289 patients across the United States and Europe. The average usage was 20 h a day for about three months, in patients either awaiting cardiac transplants or with a recent myocardial infarction or coronary artery bypass surgery, and an increased risk of sudden cardiac death. Temporary skin rash was the only major side effect noted.
While the original WCD was a monophasic device, a biphasic device was tested for acute termination of VF by Reek et al.7 The biphasic device had a maximum output of 150 J and it could terminate induced VF at the first attempt with 70 J in 12 and with 100 J in 10 episodes tested. Thus it would provide an adequate safety margin for defibrillation, though the authors recommended programming maximum energy output for ambulatory WCD patients.
As per the manufacturer's website, over 100,000 patients have been using the WCD by July 2013, with a first shock success rate of 98%. Inappropriate shocks were less than one per month of use and the shock event survival was 92% (conscious on arrival at the emergency department or remained at home). Median daily use has been 22.5 h per day.8 Rockstar free download reece mastin x.
3. Technical aspects
3.1. Components of a WCD system
The WCD system has three defibrillation and 4 ECG sensing electrodes, fitted within a garment to be worn by the patient. The defibrillation electrodes are self gelling type and the ECG electrodes are non-adhesive dry tantalum oxide capacitive electrodes. The defibrillator unit is carried on a waist belt (Figs. 1 and and2).2). Two ECG channels can be monitored with the two pairs of ECG electrodes from front to back and right to left lead sets.9 Microampere alternating current is used to check electrode contacts as in conventional monitoring systems.
Components of a WCD system (Courtesy, ZOLL).
Zoll Life Vest Manual Download
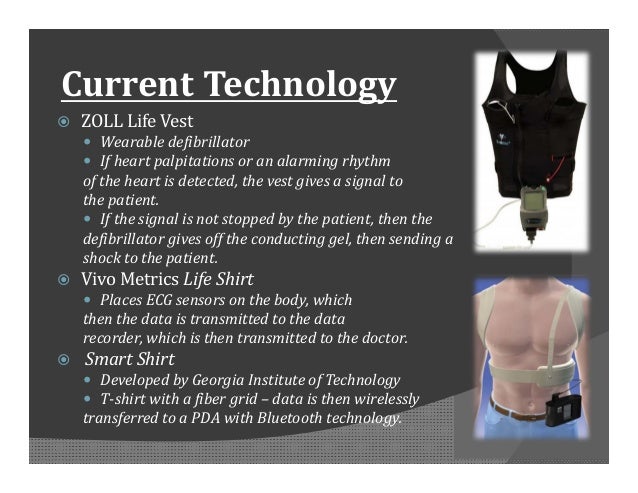
Typical method of applying the life vest (Courtesy, ZOLL).
3.2. Functioning of WCD system
The system uses heart rate, template matching and the event persistence before deciding on defibrillation. There is a sensing function to detect electrode fall off as it is used in externally worn electrode systems. If the signal from one lead is found to be suboptimal, the device will revert to single channel mode, ignoring the inputs from the faulty channel. A patient responsiveness system allows aborting of defibrillation attempt in a conscious patient. Patient responsiveness system gives out a vibratory alarm once the arrhythmia is detected. This is followed by a cascade of audible alarms of increasing intensity so that the patient has the option to press the patient response button to avert a shock. Just before delivering the shock, the defibrillation electrodes release a gel to reduce the electrical impedance and the device gives an announcement for bystanders to keep off the patient. If the patient does not respond to the alarms or the response button is released by an unconscious patient the system delivers 5 shocks. ECG records from 30 s prior to the alarm until 15 s after the alarm can be stored and sent to a secure server by modem later. Patients can also initiate manual ECG recordings.9 If the WCD detects an asystole, it gives an announcement to call the ambulance so that bystanders can respond.
4. Clinical trials of WCD
The data from the Wearable Defibrillator Investigative Trial (WEARIT) and Bridge to ICD in Patients at Risk of Arrhythmic Death (BIROAD) trials on 289 patients was published in 2004.10 WEARIT enrolled 177 patients with symptomatic heart failure and LVEF less than 30% while BIROAD Study enrolled 112 patients after myocardial infarction or coronary artery bypass grafting (CABG) at high risk of sudden death, but not receiving an ICD upto 4 months. The studies were designed as separate studies, but on request of the FDA the results had to be reported using the pooled data from both studies. There occurred 8 VT/VF episodes in 6 patients, six episodes (75%) were successfully treated with the first shock by the WCD. In over 901 months of patient use, six inappropriate shocks occurred (monthly rate of 0.67%). Six of the 12 deaths which occurred during this period were sudden deaths. Of these 5 occurred when the patients were not wearing the WCD while one occurred while the device was been worn incorrectly. Though in general the device was well tolerated, 68 patients stopped using it due to comfort issues or adverse reactions.
5. Real world data on the use of WCD
Dillon et al9 performed a retrospective analysis of arrhythmia detection, appropriate and inappropriate shocks over a one year period. A total of 2105 patients wore the WCD between January 1, 2006, and December 31. Twenty one percent used it following an acute myocardial infarction, 10% had old myocardial infarction, 28% cardiomyopathy and 21% used it following ICD removal. Combined WCD usage period for all patients was 102,583 days (3419 patient-months). The median period of usage was 36 days (range of 3–365) and the median daily use was 21.3 h (range of 0–23.9). The rate of appropriate shocks was 1.58 per 100 patient-months while that of inappropriate shocks was 0.99 per 100 patient-months. Only 2.7% of the arrhythmias detected lasted more than 25 s, while the majority was self-terminating.
A post market release nationwide registry from the United States captured the data of 3569 patients using WCD.11 The usage period ranged from 1 to 1590 days. Average daily use of more than 90% of time was noted in 52% of patients and more than 80% usage in 71% of patients respectively. Fifty nine patients had 80 VT/VF episodes and first shock was successful in 79 of 80 episodes (99%). This corresponds to an event rate of 20 appropriate shocks per 100 patient years. Interestingly, in the patient in whom WCD could not terminate VT, even multiple external shocks from the ambulance and in the emergency department could not terminate the arrhythmia that was finally converted pharmacologically. Eight patients who were unconscious with VT/VF finally died even after successful cardioversion of the arrhythmia by the WCD. Overall survival was 99.2% (3541 of 3569 patients). Survival for VT/VF events was 90% (72 of 80). There were 341 patients with early post infarction left ventricular dysfunction (‘window period’) in which an ICD is not recommended based on current guidelines.12 Ten of them experienced an arrhythmic event with appropriate WCD shocks and eight survived. Discontinuation of WCD use was primarily due to comfort issues (size and weight of the device) which occurred in 14.2% of patients.
5.1. Use of WCD in children
Data on the usage of WCD in children are scarce. Everitt et al13 reported the data on four children below the age of 18 years who used WCD during a two and a half year period at their center. None of them had an appropriate shock, but two of them had non compliance in usage. This lead to failure of detection and treatment of life threatening arrhythmia in one of them. Two patients needed down sizing to improve electrode contact and sensing.
Collins and colleagues used the manufacturer's database for a retrospective analysis of usage in children of age 18 years or less and compared it with data on those between the ages of 19–21 years.14 In the first group they could identify 81 patients, while there were 103 patients in the latter group. They found no differences between the groups in the average hours of usage per day or the total number of usage days. In the younger patient group, there was one inappropriate therapy and one device–device interaction leading to withholding of therapy. No patient received appropriate shocks in the younger age group, whereas two patients had five appropriate discharges and one patient had an inappropriate discharge in the older group.
5.2. WCD in congenital heart disease
43 patients with congenital heart disease received a WCD between 2005 and 2010 as per the prospective nation wide WCD registry from the United State.15 Their mean age was 38 ± 27 years and 37% of them had LVEF less than 30%. The indication for usage was mainly transplant listing and there was 91% compliance in the usage of WCD. 37% had tetralogy of Fallot while 21% had a combination of other lesions. Solitary lesions were present in 29%. No significant arrhythmias were documented during the median usage of 27 days and there were no appropriate or inappropriate shocks. There was 5% mortality during WCD use and 8% in the one year follow up after WCD use contributing to a total of 13% in the study period.
5.3. WCD in inherited arrhythmias
The indication for WCD usage in 119 patients with inherited arrhythmias was mainly while waiting for the results of genetic testing (47%) and following explantation of an ICD for infection/malfunction (30%).15 54% of patients had long QT syndrome, 33% had Brugada syndrome, and 8% had arrhythmogenic right ventricular dysplasia (ARVD). WCD shocks successfully corrected arrhythmias in three cases during a median usage period of 29 days and there were 7 inappropriate shocks. This corresponds to event rates of 27 appropriate shocks per 100 patient years and 63 inappropriate shocks per 100 patient years. Inappropriate shocks were due to noise being detected as VT/VF. Compliance rate of wearing WCD was 91%. There was 2% mortality during WCD usage (one patient was not wearing the device at the time of death and the other one died of peritonitis following colonic rupture) and 1% in the following year.
5.4. WCD in peripartum cardiomyopathy
107 women in the age group of 17–43 years used the WCD between 2003 and 2009 for peripartum cardiomyopathy.16 Of these 13 (12%) women used it during pregnancy, while the rest had it after delivery. No appropriate or inappropriate shocks were documented during an average usage of 124 ± 123 days. No patient died during WCD usage. Mean daily usage was 18.3 ± 5.3 h (76.3% daily usage). Discontinuation of usage was due to non-adherence or discomfort in 14%.
6. Indications for a WCD
The WCD is designed to bridge a temporary risk of sudden arrhythmic death until ICD implantation or when the risk can not be determined yet (i.e. newly diagnosed cardiomyopathy or myocarditis). Following situations can be considered as indications for WCD use:
To prevent sudden arrhythmic death until the indication for ICD implantation is clearly established as after a recent myocardial infarction or coronary artery bypass grafting or until significant reduction of risk had occurred.
- 2.
Patients before or immediately after percutaneous or surgical revascularization, when improvement of left ventricular function can be expected.
Patients awaiting cardiac transplantation, as an alternative to ICD implantation.
- 4.
When temporary protection is needed as in patients after explantation of an infected ICD system awaiting reimplantation.
Temporary inability to implant an ICD due to comorbid conditions.
- 6.
Patient refusal of ICD which is clinically indicated.
Patients with ICD indication, who are in NYHA class IV heart failure or have a life expectancy of less than year.
Indications for use as per medical orders available from the manufacturer's website8 (July 2013) is given in Table 1.
Table 1
| Indication | Percentage |
|---|---|
| Recent MIa | 26 |
| Recent CABGb | 9 |
| Early NICMc | 37 |
| Class IV CHFd | 2 |
| Explants | 8 |
| Sudden cardiac arrests | 16 |
| Genetic | 1 |
| Unspecified | 1 |
7. Inappropriate WCD shocks
Just like ICDs, WCDs can also give inappropriate shocks when actually one is not needed. The various reasons include noise detecting in the ECG signal, supraventricular tachycardia, pacemaker spikes, double counting due to T wave oversensing and non-sustained VT. Of these, the most common are signal artefacts. An inappropriate shock rate of 0.67–1.4 per 100 patient-months usage has been reported.9, 10, 11 Unlike in ICD recipients, the patient wearing a WCD can withhold an inappropriate shock by pressing the patient response button Therefore inappropriate shocks by the WCD occur only when there is a combination of inappropriate detection and absence of patient response. In fact the inappropriate shock rates were five times lower than the inappropriate detection rates. The absence of patient response could be due to various reasons including mental/physical inability at that time, forgetting the training given and rarely due to not recognizing the alarm.
8. Potential problems specific to WCD
Unlike ICDs, the WCD is worn externally and sensing problems can occur due to deformation of skin and movement of the electrodes over the skin. Skin burns under the defibrillation pads have been reported rarely. Electromagnetic interference and interference from non-cardiac electrical signals can affect the sensing function of the WCD and may cause inappropriate shocks.9 Theoretically, external electromagnetic influences can potentially affect the WCD more than the ICD due to the external location of the ECG monitoring electrodes. An inappropriate shock given due to signal interference can potentially induce ventricular fibrillation as it may not be synchronized correctly. Though the WCD attempts to synchronize the shock to the R wave for a period of 3 s, synchronization may not be possible if there is severe interference on the sensed ECG signal. Unsuccessful defibrillation due to incorrect reversal of electrodes by patients in such a way that the shocks were not directed towards the skin has occurred in two patients.10 This problem has been resolved in the current version of WCD which gives an alarm if the skin contact of the defibrillation electrodes are not proper. Non-compliance has decreased from 24.5%10–14.2%11 mainly due to a 40% decrease in size and weight of the defibrillator unit and new chest garments. It is known that some patients may have asystole or severe bradycardia after defibrillation for VT/VF. As up to now, pacing capabilities are not available in the WCD.
9. Device–Device interactions
Life Vest Nursing Instruction
LaPage MJ17 reported a fatal device–device interaction between a unipolar pacemaker and a WCD. In an 18-year-old awaiting cardiac transplantation, ventricular tachycardia was initially detected correctly by the WCD, but the device later reverted to non-recognition mode due to detection of large unipolar pacing artifacts, therapy was not delivered culminating in patient demise.
10. The future of WCD
Future technological advances are sure to bring down the size and weight of the WCD further and make its use more patient friendly. Backup bradycardia pacing would be desirable, but will probably be difficult to achieve. Electrode noise reduction and improvement in signal to noise ratio can bring down the rate of inappropriate detections and shocks significantly. WCD rental services can also enhance the usage rates by bringing down the cost and making it more affordable even in developing countries. Good patient education and training will enhance the compliance rates. Clinical registries and studies will have to define which patients will have the greatest benefit. One of the major questions to be addressed is, if bridging a temporary risk after myocardial infarction or CABG using the WCD can improve later risk-stratification for prophylactic ICD implantation.
Conflicts of interest
All authors have none to declare.